- Glucose was detected in the cellobiose grown culture, but not in cellulose-grown cultures, suggesting that cellobiose is hydrolyzed extracellularly rather than being directly assimilated, and that cellulose may not be degraded via cellobiose. Also, cellobiose-based cultures caused greater acidification of the medium than glucose or cellulose grown cultures.
- C. hutchinsonii produces and releases slimy substances when cultured in liquid, making the medium viscous. Slime production in C. johnsonii was reported to increase with culture maturity [Follett & Webley 1965]. The slime is reported to consist of polysaccharides composed of glucose, mannose, arabinose, xylose and glucuronic acid residues [Martin et al. 1968].
Cells in cellobiose-DSM3T medium might hydrolyze cellobiose first, releasing glucose, and then use glucose for growth. This suggested that cellobiose was not assimilated directly by cells. But the cells can't turn cellulose into cellobiose. Also, the cellobiose culture showed that almost no other mono- or oligo-saccharides were produced in the culture.
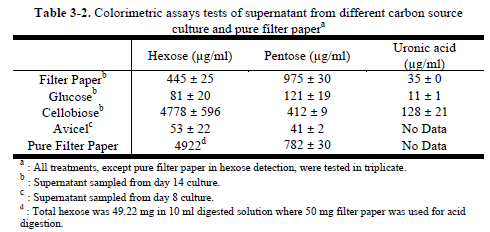
The spots from the filter paper culture did not exactly match any of the sugar standards; however, the color matched the color of the xylose and arabinose standards. Also, oligosaccharides composed of pentose. Hexoses and pentose accumulated slowly in cellulose (filter paper) culture
- Cell growth with filter paper was observed after 3 or 4 days as translucent yellow areas on the filter paper strips.
C. hutchinsonii degraded filter paper completely and produced translucent area with yellowish slime.
Cells did not transfer to adjacent filter paper strips which suggested that they were unable to cross intervening agar surfaces. Degradation could be initiated either from the edge or the interior surface of filter paper strips. The plates could be maintained by putting new filter paper strips on the yellow areas.
The ability of cells to spread over agar plates supplemented with glucose or cellobiose, but not between filter paper strips on plates lacking such supplementation, suggests that gliding is associated with substrate uptake or requires constant energy input
- The maximum cell density achieved on filter paper was much lower than that seen with glucose. The growth in filter paper cultures gradually increased from day 3 and stabilized after day 9 at OD.
- Colorimetric assays for metabolic product determination: Colorimetric assays are widely used for quantification of monosaccharides, deoxy-sugars and uronic acids. The general concept is based on forming furfural or related chemicals in strong acids, sometimes with heating
high-performance liquid chromatography (HPLC)
- EPS (Extracellular polymeric substances) from C. hutchinsonii could be utilized by Bacillus subtilis and maintained growth of B. subtilis in a mixed culture with cellulose as sole carbon source.
- if we want it to make glucose for e coli to use, and we want to start with cellulose instead of cellobiose, we may wanna consider pairing it with a microbe that can turn cellulose into cellobiose since C. hutchinsonii releases glucose when grown on cellobiose (it is not directly assimilated)
- C. hutchinsonii is capable of completely degrading crystalline cellulose
- almost 70 % of CMCase (carboxymethyl cellulose) activities were in the periplasm of C. hutchinsonii
-
the cellulose degradation system in C. hutchinsonii may be different from those of previously studied microorganisms; thus, carbohydrate binding and transport is interesting.
Many researchers believe this bacterium may have a unique cellulose-degrading mechanism which is different from either the free cellulase system or the cellulosome complex [Wilson 2008, Xie et al. 2007].
- "Most putative ß-1,4-endoglucanases of C. hutchinsonii are presumably extracellular or outer membrane associated... However, even with all the theoretical expectations, the exact cellulose degrading mechanism is still unclear."
- Direct adhesion is needed for C. hutchinsonii to hydrolyze cellulose, but no obvious encoded protein has been found relating to adhesion [Xie et al. 2007].
- Insertion of plasmids directly to C. hutchinsonii has been reported by Xu et al. (2011). McBride and Baker (1996) have reported transformation of C hutchinsonii by conjugation.