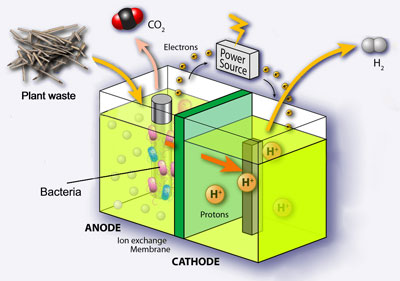
- One approach: using Caldicellulosiruptor saccharolyticus
- Gram-positive anaerobic bacterium that ferments a broad spectrum of mono-, di- and polysaccharides to mainly acetate, CO2 and hydrogen
- hydrogen yields approaching the theoretical limit for dark fermentation of 4 mol hydrogen per mol hexose
- organism has proven itself to be an excellent candidate for biological hydrogen production
- ability to produce thermostable cellulolytic and xylanolytic enzymes, to grow on complex lignocellulosic carbon sources, and to co-metabolize a wide spectrum of monosaccharides including both pentose and hexose sugars
- factors to consider: hydrolytic capability, sugar metabolism, hydrogen formation, mechanisms involved in hydrogen inhibition, regulation of the redox and carbon metabolism
- challenges/drawbacks:
- growth medium
- growth temperature
- Coupling hydrogen production with other processes
- formate production to fuel hydrogen production (iGEM Tokyo 2012)
- using hydrogen production in a bacteria in conjunction with another bacteria or system that would allow cheaper or more efficient production of another useful molecule
- hydrogen produced in conjunction with an electroconductive bacteria (Geobacter - Nick works with it in his lab) to immediately produce electricity
- Modify the endogenous activator of the operon and up-regulate it to increase hydrogen production
- Using a new gas separation process to remove/purify the hydrogen
- concept of "consolidated bioprocessing"
- one bacteria is able to produce cellulase, hydrolyze cellulose, and ferment in one step
- This increases efficiency decreases total cost, and removes the requirement for pre-treating the biomass.
- Putting multiple hydrogen production pathways in each bacterium
- hydrogenase, plus formic acid, plus etc.
- Using a moderate thermophile
- Open-ended questions for next week:
- pros of using E. Coli
- lots of models and tools to use
- There are established ways of manipulating central metabolism of E. Coli (i.e. to produce formate)
- Metabolic flux engineering - professor Graffner?
- Pam Silver - talk to people in her lab; may have been working with geobacter
- MAGE = multiplex automated genome engineering
- introduce point mutations to specific places
- directed evolution (but not easy to assess)
- How to assess hydrogen production
- pubmed: intracellular hydrogen sensors
- continuous directed evolution
- How to get electricity of geobacter out of the flask
- fuel cell has already been developed (uses sediment and water)
- Come up with a more concrete plan
- using 2 specific bacteria and coupling their metabolic processes
- possible sybio approach: improving communication between the cells
- another possible approach: controlling when it produces with certain inputs
- pros of using E. Coli
| link to article | summary/important points |
|---|---|
| http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3003633/ | background information about Caldicellulosiruptor saccharolyticus |
| http://2010.igem.org/Team:ULB-Brussels/H2 | goal: increase the H2 production by modifying the carbon flow through the pathway of the mixed acid fermentation |
| http://2012.igem.org/Team:UT-Tokyo | formic acid |
| http://www.sciencedirect.com/science/article/pii/S0360319906002205 | Integration of biohydrogen fermentation and gas separation processes to recover and enrich hydrogen |
| http://www.sciencedirect.com/science/article/pii/S0960852413006093 | Consolidated bioprocessing of untreated switchgrass to hydrogen by the extreme thermophile Caldicellulosiruptor saccharolyticus DSM 8903 |
| http://www.biotechnologyforbiofuels.com/content/7/1/82 | Single-step bioconversion of lignocellulose to hydrogen using novel moderately thermophilic bacteria |
| http://www.sciencedirect.com/science/article/pii/S0141022901003945 | Bioreactor structures of bacterial H2 production |
| http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2636881/ | Increased H2 production by e coli |