Overview
Composite Propellants use a polymer binder to hold their constituent components in place. The composite binder has a curing process that chemically results in the linking of chains of molecules via a dehydration reaction . To enable this reaction a curative agent needs to be added. In order to see the best mechanical properties in the cured propellant all of the possible cross linking sites should be linked. This would require that the same number of donating sites and receptor sites exit. Many chemicals in a generic composite propellant formulation can interact with the polymerization. The way to handle this interaction is with a value known as the equivalent weight. Equivalent weight accounts for the relative number of linking sites accepted or donated, and the relative mass of a compound.
Software
This formula uses the equivalent weight of the binder, curative, and castor oil to calculate the fractions of HTPB and curative, given the sum of HTPB/curative and fraction of castor oil.
The following python file will walk you through this calculation.
Balancing the Equivalent Weight of a Formulation
A succinct overview of correctly determining the proper amount of curative for a given formulation was done by John DeMar, and is linked below.
https://wikis.mit.edu/confluence/download/attachments/122621517/Curative_calculation.pdf?api=v2
Chemistry
The polymerization process of HTPB involves the reaction of OH functional groups with NCO functional groups. The HTPB rubber provides the OH sites for the cross linking, which starts when the isocyanate curative is added.
The structure of HTPB is below:
"m" and "n" are arbitrary integer values, with both ends of the molecule capped with an OH group.
A common curative is Isophorone diisocyanate (IPDI). It's structure is shown below:
A given molecule of IPDI will chain two molecules of HTPB together.
Because there are only two sites on both molecules, the resulting polymer would be one long chain (or more likely, several distinct, but shorter ones). To improve the mechanical properties of the resulting polymer, cross linking agents are added. Graphic courtesy of Professor Jeff Grossman (3.091)
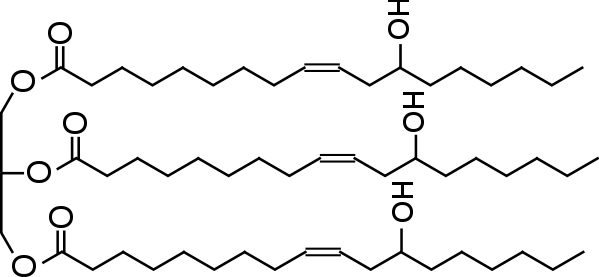
A typical cross linker is castor oil which has 3 cross linking sites. Structure below:
Adding two molecules of castor oil requires the addition of three molecules of isocyanate. Castor oil is also a heavier molecule than most curative molecules.
Other compounds can also add or subtract from the number of curative molecules required for a complete cure. Because the curative molecules are usually much lighter than the hydroxl terminated molecules, it is typical to see curatives as approximately 2.5% by mass of a propellant mixture.