PROTEIN RECEPTOR GROUP
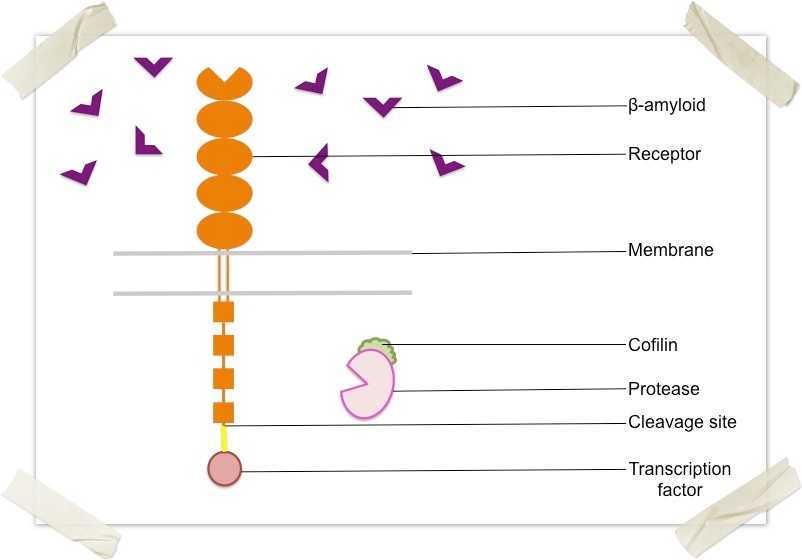
The plaques found in the brain of a patient with Alzheimer's disease comprise beta-amyloid protein oligomers, which are responsible for the degenerative symptoms of the condition. In order to diagnose and/or treat Alzheimer's disease, we must first be able to detect the presence of these beta-amyloid oligomers. One of the possible methods of detection is the use of beta-amyloid oligomer-specific transmembrane receptors. Leukocyte immunoglobulin-like receptor (LilrB2) and its murine homolog Paired Immunoglobulin-like Receptor B (PirB) are trans-membrane receptors capable of selectively binding beta-amyloid oligomers (of x or more monomers). It belongs to a family of proteins that bind to MHC1 molecules on antigen presenting cells and is only expressed in monocytes and B-cells (and at lower levels in dendritic cells and natural killer cells) in humans. When beta-amyloid oligomers bind to the extracellular domain of LilrB2, it is activated and then goes on to recruit cofilin to its intracellular domain. In our proposed detection model, we would construct a fusion protein where a linker, TEV protease cleavage site and transcription factor (in that order) are fused to the intracellular domain of LilrB2. We would also fuse a TEV protease to cofilin. In that way, when beta-amyloid oligomers bind to the receptor, thus activating it, the TEV protease on the recruited cofilin cleaves at the cleavage site releasing the transcription factor in to the cytosol. The cleaved portion will be guided to the nucleus of the cell and will go on to activate the subsequent processing module. |
Experiments: | |
|
|---|
TO DO (cloning things): | |
| 8/18 |
|
|
| 8/21 |
| |
For LilrB2TCS only:
| 8/28
8/29
|